
Clostridioides difficile – Antigen and PCR
Consistent with O. Reg. 671/92 of the French Language Services Act, laboratory testing information on this page is only available in English because it is scientific or technical in nature and is for use only by qualified health care providers and not by members of the public.
Background:
This page provides routine antigen and polymerase chain reaction (PCR) testing information for Clostridioides difficile infection (CDI) at Public Health Ontario (PHO). Manifestations of CDI range from mild diarrhea to severe and fulminant disease (e.g. pseudomembranous colitis, toxic megacolon).
This page is for information limited to routine primary CDI testing. For cultured isolate typing information of confirmed cases to assist with outbreak investigations, please refer to the following PHO webpage:
Clostridioides difficile – Culture and Pulse Field Gel Electrophoresis (PFGE) Typing
Updates:
As of October 18, 2023, the following updates have been added:
- Integration of the information previously under labstract LAB-SD-002
- Change in molecular confirmation method with the BD MAX Cdiff assay replacing the Alethia C. difficile assay
- Graphical changes to the stool consistency chart
Testing Indications
As per the IDSA/SHEA guidelines, C. difficile testing is indicated for individuals with unexplained diarrhea (e.g. ≥ 3 unformed stools per day without underlying diarrheal condition such as laxative use) and with risk factors of C. difficile disease (e.g. systemic antibiotic therapy, hospitalization, advanced age, impaired immunity, gastrointestinal surgery).1
Caution #1: C. difficile testing is not indicated for asymptomatic individuals due to the possibility of clinically insignificant toxigenic C. difficile excretion in healthy individuals. Similarly, testing is not indicated in patients under 12 months of age with diarrheal symptoms due to the high rate of concomitant C. difficile colonization in healthy newborns and infants. Otherwise, C. difficile testing in children with diarrheal symptoms should only be requested if there is a strong suspicion of CDI in the absence of other possible etiologies.
Caution #2: Repeat testing to monitor treatment response (or ‘test of cure’) is not indicated, as test results may remain positive for weeks to months despite resolution of infection.
Acceptance/Rejection Criteria
Testing requests will be rejected in the following scenarios:
- Formed stool specimens (refer to the stool consistency chart for acceptable stool types)
- Rectal swabs
- Specimens in transport or preservative media (e.g. Cary Blair, formalin, SAF, PVA)
- Specimens from patients under 12 months of age
- Insufficient specimen volume submitted (> 10 ml of stool required) or leaking specimen
- Multiple specimens collected on the same date for the same patient
Specimen Requirements
| Test Requested | Required Requisition(s) | Specimen Type | Minimum Volume | Collection Kit |
C. difficile |
Unformed stool |
10 ml |
Empty sterile container |
Submission and Collection Notes
See Special Instructions below for recommended stool collection method.
Complete all fields of the requisition form, including:
- Test(s) requests and indications for testing
- Patient setting/population
- Clinical information including symptom onset date
Label the specimen container(s) with the patient’s first and last name, date of collection, and one other unique identifier such as the patient’s date of birth or Health Card Number. Failure to provide this information may result in rejection or testing delay.
Limitations
Only one sample per patient is tested per collection date.
Storage and Transport
Specimens should be stored at 2-8°C following collection and shipped to PHO’s laboratory on ice packs. Specimens must be received within 72 hours of collection for optimal testing. All clinical specimens must be shipped in accordance to the Transportation of Dangerous Good Act.
Note: Freezing of the specimen is not required. Final test results may be affected by freezing and thawing and may not be accurate.
Special Instructions
The following stool specimen collection method is recommended:
- Uncap the empty sterile container.
- During a bowel movement, collect a sufficient amount of unformed* stool in a clean and dry receptacle (e.g. Chamber pot, wide-mouth jar, pie plate, or cellophane wrapped around toilet bowl) while avoiding urine or water coming in contact with the stool. (*Note: refer to the stool consistency chart for acceptable stool types.)
- Using a disposable clean stick or spoon, transfer a minimum* of 10 ml of unformed stools. (*Note: do not overfill).
- Recap the container tightly and shake vial to check that there is no leakage.
- Label the container with the patient’s full name, date of collection, and date of birth or health card number.
Test Frequency and Turnaround Time (TAT)
Clostridioides difficile antigen testing and confirmatory molecular testing are performed Monday to Saturday at PHO’s laboratory.
Turnaround time is up to 24 hours for antigen testing and up to 3 business days for confirmatory molecular testing from receipt at PHO’s laboratory.
Clostridioides difficile antigen testing is performed by enzyme immunoassay (EIA) using either the TechLab C. DIFF CHEK-60 (PHO’s Toronto laboratory site) or the TechLab C. DIFF QUIK CHEK COMPLETE (all other PHO laboratory sites) for the detection of glutamate dehydrogenase (GDH) (CHEK-60) or GDH in combination with toxins A and B (QUIK CHEK COMPLETE). The difference in test methodology for antigen detection was selected based on the volume of specimens received at each laboratory site within PHO.
Clostridioides difficile confirmatory molecular testing is performed by qualitative PCR using the BD MAX Cdiff assay for the detection of the toxin B (also known as cytotoxin) gene.
Performance and limitations
GDH antigen testing evaluates the potential presence of C. difficile bacteria in stool. It has a reported sensitivity of 94-96% and specificity of 92-95% compared with reference methods (e.g., cell cytotoxin assay or cytotoxigenic culture)2. Negative results usually rule out CDI but positive results may represent nontoxigenic C. difficile excretion and therefore require confirmation with either toxin antigen testing or toxin gene PCR testing.
Toxin antigen testing evaluates the presence of toxin A and B in stool but does not distinguish between either toxins. It has a reported sensitivity of 58-83% and specificity of 99% compared with reference methods. Positive results usually correlate well with clinically significant CDI but negative results do not rule out C. difficile due to the limited standalone assay sensitivity. When combined with parallel GDH antigen testing without PCR testing, it has a reported sensitivity of 58-82% and specificity of 99.5%.
Toxin B PCR testing evaluates the presence of toxigenic C. difficile genetic material in stool. When following a positive GDH antigen test result, it has a reported combined sensitivity of 91-96% and specificity of 96-98% compared with reference methods. In some instances, positive results may represent toxigenic C. difficile excretion without clinically significant toxin production, therefore results should always be correlated with clinical findings and the absence of other potential diarrheal illness etiologies.
Algorithm
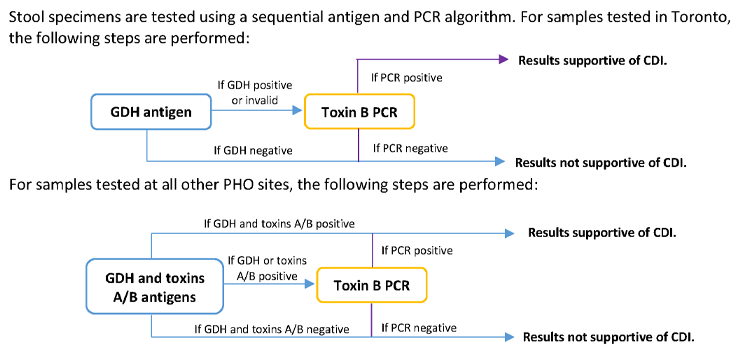
Interpretation
The following table provides possible test results with associated interpretations:
| GDH Antigen | Toxins A/B Antigen | Toxin B PCR | Interpretation |
|---|---|---|---|
| Not detected | Not performed or not detected | Not performed | Results are NOT supportive of toxigenic C. difficile infection. |
| Not detected or invalid | Detected or invalid | Not detected | Results are NOT supportive of toxigenic C. difficile infection. |
| Detected or invalid | Not performed, not detected, or invalid | Not detected | Results are NOT supportive of toxigenic C. difficile infection. |
| Detected or invalid | Not performed, not detected, or invalid |
Detected | Results are supportive of toxigenic C. difficile infection but must be interpreted with clinical findings, as asymptomatic carriage may also occur. Note: repeat testing to monitor treatment response is NOT indicated. |
| Detected | Detected | Not performed | Results are supportive of toxigenic C. difficile infection but must be interpreted with clinical findings, as asymptomatic carriage may also occur. Note: repeat testing to monitor treatment response is NOT indicated. |
| Not detected or invalid | Detected or invalid | Detected | Results are supportive of toxigenic C. difficile infection but must be interpreted with clinical findings, as asymptomatic carriage may also occur. Note: repeat testing to monitor treatment response is NOT indicated. |
| Invalid | Not performed or invalid | Invalid | Testing is invalid which may be due to interfering substances in the specimen. Repeat collection and testing is recommended if clinically indicated. |
Reporting
Results are reported to the physician, authorized health care provider (General O. Reg 45/22, s.18) or submitter as indicated on the requisition. Note: positive results are not routinely reported to the Medical Officer of Health (MOH), therefore submitters suspecting a C. difficile outbreak in their hospital facility have the responsibility to report the outbreak to their facility’s MOH.
References
- McDonald LC, Gerding DN, Johnson S, Bakken JS, Carroll KC, Coffin SE, Dubberke ER, Garey KW, Gould CV, Kelly C, Loo V, Shaklee Sammons J, Sandora TJ, Wilcox MH. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018 Mar 19;66(7):e1-e48. doi: 10.1093/cid/cix1085.
- Planche TD, Davies KA, Coen PG, Finney JM, Monahan IM, Morris KA, O'Connor L, Oakley SJ, Pope CF, Wren MW, Shetty NP, Crook DW, Wilcox MH. Differences in outcome according to Clostridium difficile testing method: a prospective multicentre diagnostic validation study of C difficile infection. Lancet Infect Dis. 2013 Nov;13(11):936-45.
Don’t have a MyPHO account? Register Now

