
Top Five High Risk Practices: Recommendations and occupational health and safety responsibilities
Integrating best practices into routine care and responding to infections appropriately decreases the risk of outbreaks, elevates the general level of practice and protects the public and office staff.
The following recommendations address the top five practices that put patients and others at risk in a physician’s office. Recommendations from PIDAC's Infection Prevention and Control for Clinical Office Practice, which was developed in collaboration with the College of Physicians and Surgeons of Ontario to support those working in clinical office settings.

Lancets, glucometers and insulin pens
- Lancets must be single use only.
- Lancet hubs (holds the lancet) must be single use only.
- Insulin pens must be single patient use only.
- Blood glucose monitoring devices (Glucometers) and other blood testing devices, should not be shared between patients.
- If they must be shared, the device must be designed for multi-patient use and cleaned and disinfected after each use, per the manufacturer’s recommendation. If the manufacturer does not specify how the device should be cleaned and disinfected then the device cannot be shared.
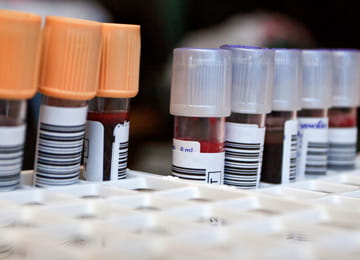
Blood Collection Devices
- Single use blood collection tube holders are preferred.
- If blood tube holder must be reused, it must be cleaned and disinfected after each use.
Tonometers
- Tonometers and other ophthalmologic equipment that touch the eye must undergo high-level disinfection (e.g., glutaraldehyde) between patient uses. Cleaning with alcohol is not sufficient.
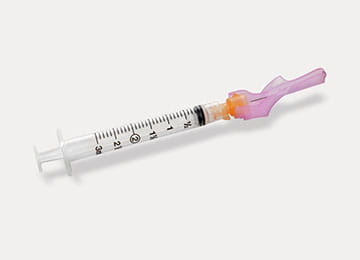
Syringes and Needles and Use of Vials for IM/IV Medications and Vaccines
- All needles are single patient use only.
- All syringes are single patient use only.
- Single use vials preferred—to be used once only on a single patient.
- When multidose vial use is necessary—never re-enter a vial with a used needle or used syringe.
- Once medication is drawn up, the needle should be immediately withdrawn from the vial. A needle should never be left in a vial to be attached to a new syringe.
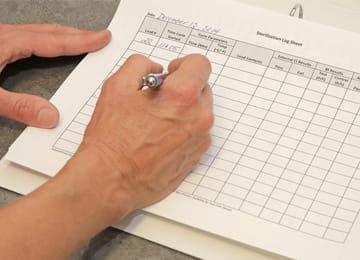
Sterilization Logs
Note: Before sterilization, meticulous cleaning must be done.
- Sterilization logs, including indicators documenting successful sterilization, are required for all office/desktop autoclaves.
- Logs must document time, temperature and pressure (physical indicators) at completion of each load.
- An external chemical indicator must be used with every packaged item to indicate the item has been processed through a sterilization cycle.
- An internal chemical indicator must be placed inside every packaged item to be sterilized.
- Daily testing of biological indicator (BI) is required when a sterilizer is in use.
- SCOPE disinfection and sterilization logs must be kept, including test strip monitoring, concentration and exposure time, and disinfectant temperature for automated endoscope reprocessors (AER).
Updated
22 Oct 2019
You need a MyPHO Account to save this page.
Log in to MyPHO
Don’t have a MyPHO account? Register Now
You have successfully created a MyPHO account!
Use MyPHO to save content relevant to you, take online courses and register for subscriptions.

